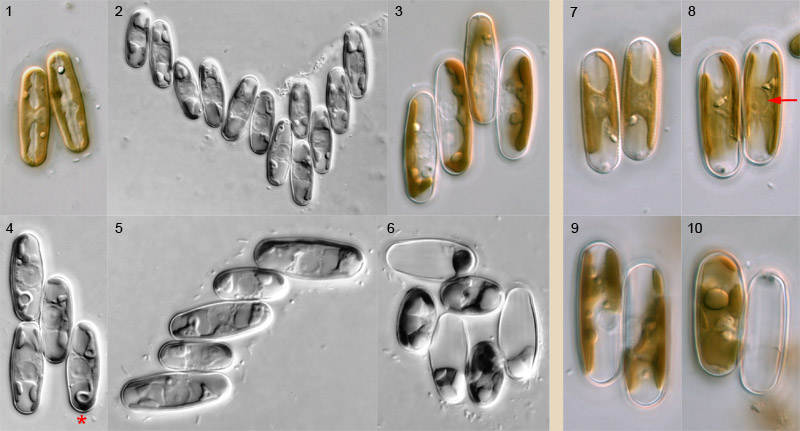
Pairing
Pairing of Sellaphora cells takes place through active movement of sexualized cells. Cells appear to be attracted to each other and move backwards and forwards close to each other before meeting and becoming firmly bonded to each other via the girdle. The capacity of cells to bond must be maintained for a considerable period (e.g. many minutes or a few hours, rather than seconds or a few minutes), since large aggregates of bonded sexualized cells can develop in dense populations of compatible sexualized cells (Fig. 2). In sparser populations, however, pairs (Figs 7–10) and triplets are the principal configurations found. Bonded cells – even in large groups like those shown – continue to move around for some time, but they become immobile before entry to meiosis. Transition to meiosis is marked by a change in the configuration of the chloroplasts and nucleus, and a marked expansion of the nucleus, which becomes ± spherical (e.g. arrow, Fig. 8).
Sexual reproduction in Sellaphora is a highly organized process that ensures that fertilization takes place between two gametes from two different gametangia. Each of the paired cells in Fig. 1 will produce a single gamete and these will fuse to produce a zygote; this process is shown on other pages.
What, then, happens when more than two cells bond together? Usually, such larger groups resolve into pairs: the configuration of the chloroplasts in Fig. 3 shows that this quadruplet has resolved itself into two pairs (in this species, S. blackfordensis, the chloroplasts 'face each other' during meiosis). The number of resolved pairs is usually close to the maximum possible, but not always. For example, the quadruplet in Fig. 4 can only produce one zygote. Why? There are three linked reasons: (1) sexualized S. blackfordensis cells are apparently always predetermined as either male or female; (2) cell–cell recognition and bonding occurs only between male and female, and (3) fertilization in S. blackfordensis occurs only via the sides of the girdle, not via the poles. Hence the central cell of Fig. 4 could potentially fertilize (if it is male) or be fertilized by (if it is female) any one of the other three, but the two cells not 'chosen' will not be compatible; even if they were (as may be the case in homothallic Sellaphora species), it would not be physically possible to achieve plasmogamy. Note too that the central cell of Fig. 4 'faces' the two left-hand cells and could potentially fuse with either; it 'has its back' to the other cell (marked with an asterisk), which has entered meiosis but apparently been rejected by the central cell in favour of the other two. Sometimes, such cells revert (and divide mitotically soon afterwards) but often they proceed through meiosis, form a gamete, and die.
In the complex cluster shown in Fig. 6, cells of two sizes are evident; these belong to two different clones that were mated in the laboratory, one clone with cells much that were much larger than the other's. It can be seen that each large cell is in contact only with small cells and vice versa. In this case, all the large cells have acted as males.
In triplets and clusters with odd numbers of cells, at least one gametangium is redundant and will die without contributing to the next generation. In Fig. 5, the configurations of the chloroplasts suggest that it is the top-most cell that is superfluous.
Pairing is similar in most species of Sellaphora that have been investigated. For example, side-by-side pairing occurs in S. blackfordensis (Figs 1–6) and S. laevissima (Figs 7–10), which belong to different clades. However, the 'urban elliptical' and 'upland elliptical' demes (names used by Evans et al., 2007 and in press) pair via any part of the cell periphery and can therefore form end-to-end and T-shaped configurations.
The photographs were taken at various times and show pairing in natural populations and two-clone cultures of S. blackfordensis from Blackford Pond and in natural populations of S. laevissima sensu lato from Lochend Loch, Edinburgh. They were taken using differential interference contrast optics, with Reichert Polyvar photomicroscopes. The cells shown are c. 15–30 µm long.

 This site is hosted by the Royal Botanic
Garden Edinburgh.
This site is hosted by the Royal Botanic
Garden Edinburgh.